CIP / SIP
Precision-engineered CIP/SIP systems ensure sterile, compliant, and efficient pharmaceutical water solutions—automating cleaning, reducing contamination risks, and validating purity to GMP standards.
CIP / SIP
Precision-engineered CIP/SIP systems ensure sterile, compliant, and efficient pharmaceutical water solutions—automating cleaning, reducing contamination risks, and validating purity to GMP standards.

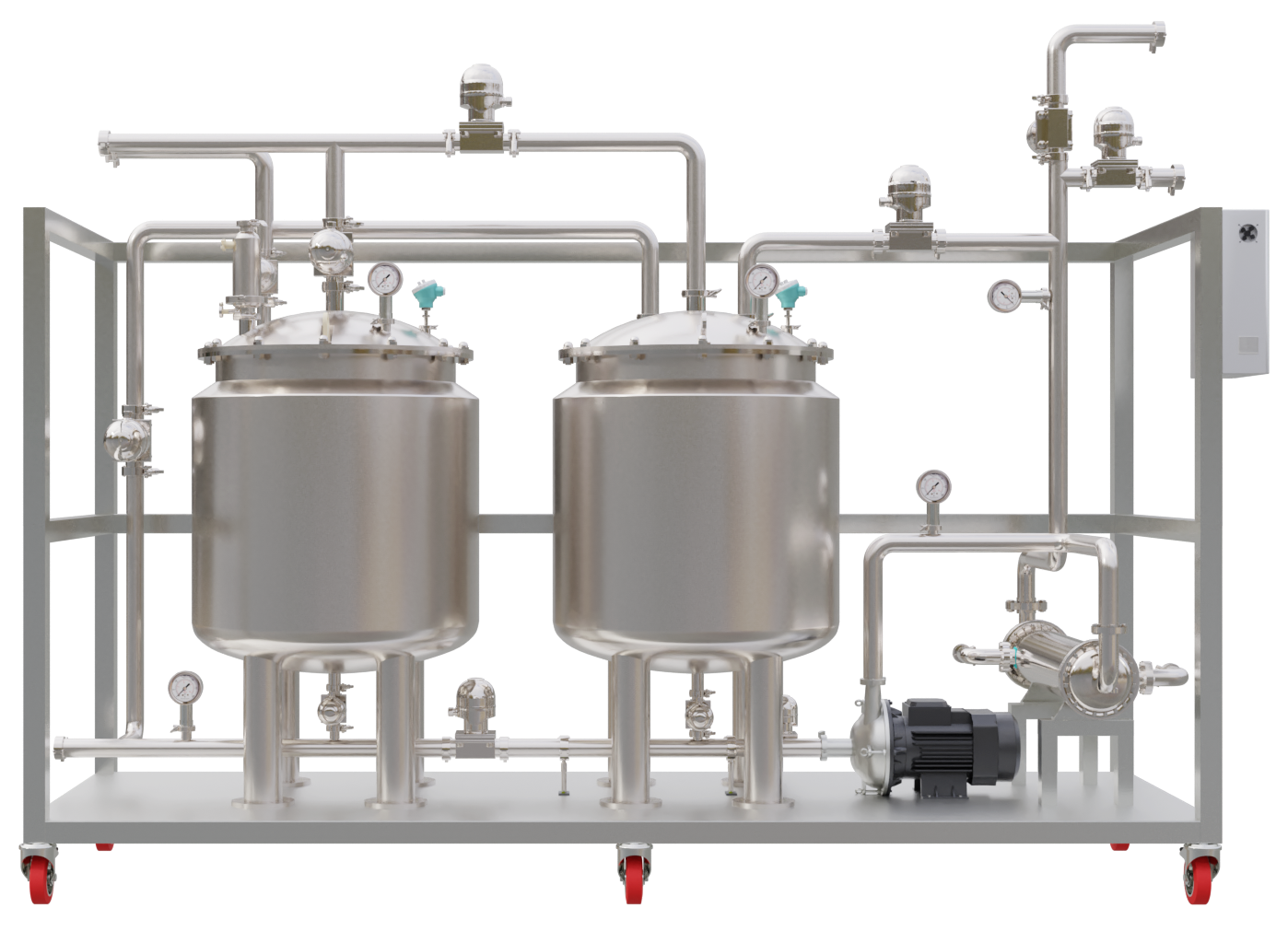
Product Description
Cleaning and sterilization are critical processes in industries where hygiene and contamination control are paramount. CIP (Clean-in-Place) and SIP (Sterilize-in-Place) systems are automated solutions designed to clean and sterilize equipment without disassembly, ensuring efficiency, safety, and compliance with industry standards.
These systems are widely used in pharmaceuticals, biotechnology, food & beverage, dairy, and chemical industries, where maintaining sterile conditions is essential. CIP/SIP systems eliminate human intervention, reducing contamination risks while saving time and operational costs.
Key Features
Automation & Compliance
Features:
- PLC/HMI-controlled with pre-validated, audit-ready cycles.
- FDA/EMA-compliant – (IQ/OQ/PQ) for GMP environments.
- 21 CFR Part 11 – Paperless data recording
- Auto-alerts – for deviations (temperature, flow, conductivity).
- Recipe Management – for multi-product facilities.
Precision Cleaning Performance
Features:
- Turbulent flow – (1.5–2.0 m/s) for full surface coverage.
- Real-time monitoring – (T, P, flow, conductivity).
- Design (ASME BPE) – Self-draining, zero dead-leg.
- Rotary jet/spray ball systems –for hard-to-reach areas.
- Low-residue verification – via TOC or conductivity checks.
Hygienic Engineering
Features:
- 316L stainless steel – with electropolished surfaces.
- Sanitary integrity – with Tri-clamp fittings and seamless welds.
- Sloped surfaces – (≥3°) for complete drainage.
- Standards – cleanable to EHEDG/SMS.
- CIP-optimized – components (valves, pumps, sensors).
Sustainability & Cost Savings
Features:
- Closed-loop chemical/water reuse to cut waste by 30%+.
- Heat exchangers – for energy recovery.
- Optimized cycles – reduce time, water, and CIP chemicals.
- Waste Neutralization – or effluent compliance.
- Predictive maintenance – to extend equipment life.
Seamless Integration
Features:
- Plug-and-play – with SCADA/MES/ERP.
- Cloud-based analytics – for remote OEE tracking.
- Mobile CIP units – for R&D or small batches.
- Multi-zone support – for large-scale plants.
- Industry 4.0-ready – (IoT, AI-driven optimization).
Sterilization Assurance (SIP)
Features:
- Steam/overkill cycles – for log-6 pathogen reduction.
- BI (Biological Indicator) testing – for sterility proof.
- Thermal mapping – with calibrated RTDs.
- Condensate management – to prevent recontamination.
- VHP (Vapor H2O2) – compatible for isolators/containment.
Key Features : (CIP)
- Automated Cleaning: Reduces manual labor and ensures consistent cleaning.
- Customizable Cycles: Different cleaning programs for varying contamination levels.
- Water & Chemical Efficiency: Optimizes resource usage, reducing waste.
- Compliance Ready: Meets GMP (Good Manufacturing Practices), FDA, and ISO standards.
Key Features : (SIP)
- High-Temperature Sterilization: Uses steam at 121°C or higher for effective microbial kill.
- Validated Processes: Ensures sterilization meets FDA, EU GMP, and USP standards.
- Reduces Downtime: Faster than manual sterilization, improving productivity.
- Data Logging & Documentation: Maintains records for regulatory compliance.
Product Description
Cleaning and sterilization are critical processes in industries where hygiene and contamination control are paramount. CIP (Clean-in-Place) and SIP (Sterilize-in-Place) systems are automated solutions designed to clean and sterilize equipment without disassembly, ensuring efficiency, safety, and compliance with industry standards.
These systems are widely used in pharmaceuticals, biotechnology, food & beverage, dairy, and chemical industries, where maintaining sterile conditions is essential. CIP/SIP systems eliminate human intervention, reducing contamination risks while saving time and operational costs.
Key Features :
Compact, Skid Mounted
Highly compact, skid mounted system, prewired and pre-tested
Portable
Designed with large castor wheels in small footprint for portability
Control & Monitoring
Monitor & control temperature, flow, concentration, cycle time setpoints etc.
PLC Control Panel
Intuitive touchscreen (HMI) enables easy selection of wash cycles
CIP/SIP
Designed for efficient cleaning and sterilization, our CIP / SIP systems are highly flexible and can be configured to suit various products and production cycles by the press of a button on the HMI
- Compact, Skid Mounted, Portable
- Large Castor Wheels, Easy Portability
- Touchscreen HMI for Easy Configuration
- Flexibility to Run Varied Cleaning Cycles
- Data Logging of All Critical Parameters
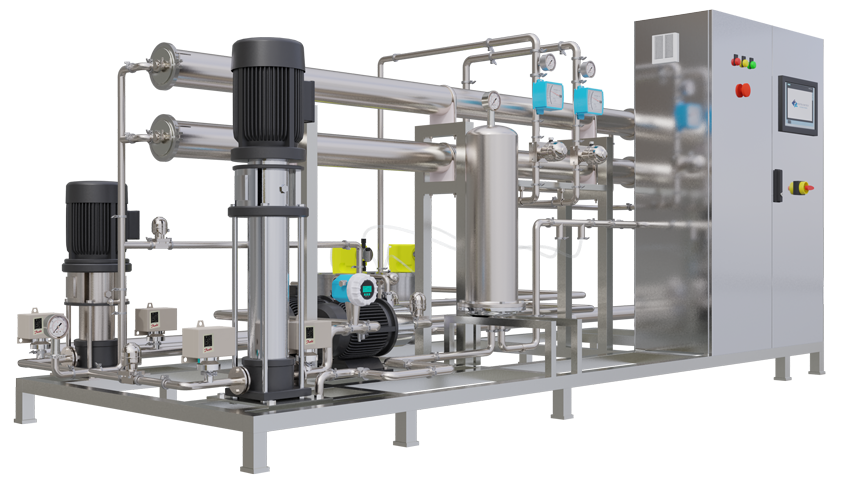
Why This System?
- Regulatory Confidence: – Built for FDA, EU GMP, WHO.
- ROI-Driven: – Slashes labor, downtime, and validation costs.
- Risk-Free: – Eliminates cross-contamination, batch losses.
- Scalable: – From pilot plants to commercial production.
- Green Manufacturing: – Reduces water, energy, and waste footprint.
Projects Executed
Our Portfolio






Schedule a Meeting
Need a customized solution for your industry? Contact us today for expert design and implementation!
Schedule a Meeting
Need a customized water solution for your industry? Contact us today for expert design and implementation!




